Document Management Software
Compliance Without Compromise – Master Document Control
Streamline compliance and gain total control over your document lifecycle. Our AI-powered document management software automates workflows, ensures version control, secure access, and provides real-time visibility—so you can focus on quality while staying audit-ready, always.
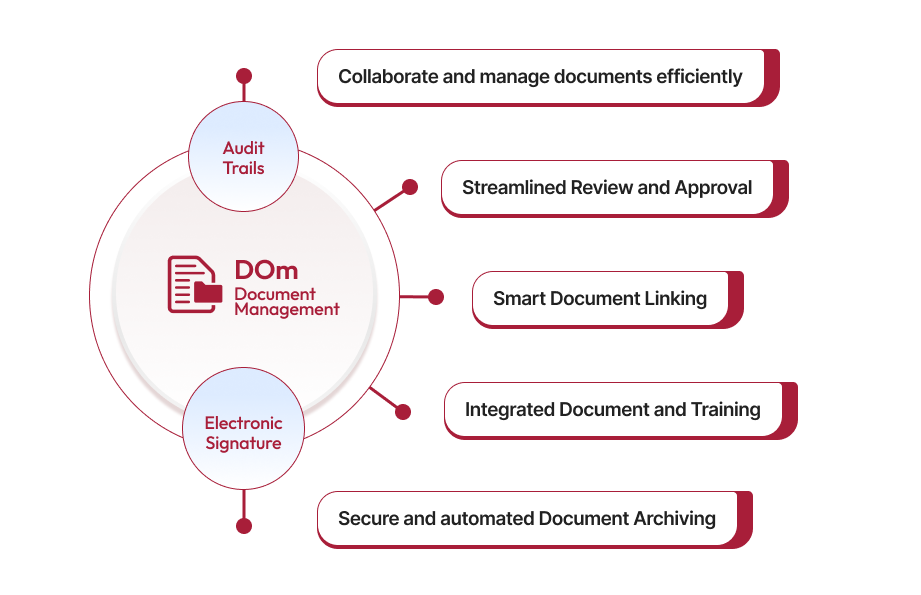
Intelligent QAI Assistant to Streamline Document Management
Qualityze Document Management solution simplifies the management of the growing volume of documents by providing a robust framework and convenient sharing and tracking capabilities. With Qualityze, you can streamline your document process and empower your team to achieve document control excellence. When you use Qualityze Document Management solution, you can ensure that your organization stays on top of its documentation needs, reducing the risk of errors and non-compliance.

Secure, Centralized Access for Smarter Collaboration
Our solution offers secure, centralized access that enhances collaboration and efficiency for your organization. With pre-defined, modifiable templates and configurable workflows, it ensures standardized, accurate document creation and management. Your teams can leverage the integration with Google Suite, or Microsoft 365, allowing global access to author, collaboration and share documents seamlessly through digital workflows.

Effortless Version Control to Keep Track of Every Edit
Never lose track of changes again. With effortless version control, you can easily monitor every edit and revision, ensuring your work is always up-to-date and organized. Collaborate seamlessly, roll back changes when needed, and maintain a clear history of your project's evolution – all with minimal effort. Stay focused on your core activities, while version control functionality takes care of the details.

On-Time Reviews and Approvals for Peak Performance
Achieve peak performance with on-time reviews and approvals, designed to streamline your document management process. With features like email approvals, alerts, task scheduling, and real-time collaboration, your team can accelerate document review and approval timelines. Easily communicate with document owners in real-time to quickly address critical changes, deviations, and nonconformances. Experience smoother workflows, reduced review cycles, and ultimate productivity with our efficient document management capabilities.

Improve Team Skills by Integrating Documents into Training
Ensure your team comprehends all the controlled documents they received with integrated document-based training. You can track understanding and performance through electronic signoffs. Our solution seamlessly connects with Change and Training Management, making document, change, and training processes closely connected and effective—crucial for compliance and business excellence.

Ensure Audit Readiness
Our system helps you stay proactive by keeping everything up to date, from document control to maintaining training records. With automated notifications, version tracking, and easy access to all necessary information, you can be confident that your team is always ready for an audit. No more scrambling for missing files or worrying about overlooked compliance gaps. Instead, you’ll face audits knowing that every requirement is accounted for, and everything’s running smoothly.

Zero Compromise on Data Integrity
Qualityze Document Management ensures that data integrity is maintained by adhering to ALCOA standards, which require all documents to be attributable, legible, complete, original or true copies, and accurate. Real-time data recording is a key aspect, ensuring that data is captured directly and not noted on temporary scraps of paper. Additionally, electronic records are safeguarded by regular backups, protecting them against potential data loss. To maintain organized document control, all documents are uniquely numbered in accordance with ORA policies.

Secure Identification with Watermark Authentication
With Qualityze, you can easily add a watermark to your controlled documents. This ensures an added layer of security and authenticity for your internal and external documents. Additionally, it prevents unauthorized edits throughout the document lifecycle.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
What Differentiates Us from Others
We have got you covered for everything you look for in a next generation document management solution to be future-ready!
- End-to-End Document Control
Manage every stage of your documents’ lifecycle effortlessly—from authoring, collaborating, revising to archiving. With our solution, you can ensure that every document is accounted for, properly stored, and always accessible when you need it. It’s all about making document control stress-free and reliable. - Configurable to Fit Your Needs
We understand that every organization has its own way of doing things. That’s why our solution is designed to be flexible. You can easily configure it as per your specific processes and preferences. You get the tools you need, tailored just the way you want them. - Built-In Compliance Validation
Staying compliant can be a challenge, but we’re here to help. Our built-in compliance features make sure that every document meets regulatory standards, giving you peace of mind that you’re always audit-ready and adheres to applicable standards. - AI-Assisted Workflows
Save time and improve operational efficiency with AI-assisted workflows that help you streamline repetitive tasks. Let our intelligent system handle approvals, reminders, and document routing, so you and your team can focus on what really matters. - Seamless Integration
Our platform goes well with others. It seamlessly integrates it with your existing enterprise tools and systems, so you won’t have to deal with the headache of switching back and forth between multiple applications. Everything you need is right at your fingertips. - User-Friendly Interface
No steep learning curves here! Our user-friendly interface makes it easy for team members to get up and running quickly. Whether it’s uploading documents, tracking changes, or generating reports, you’ll find everything straightforward and intuitive. - Continuous Improvement Culture
We believe in constantly getting better, and our smarter quality solutions reflect that mindset. With continuous updates and new features based on user feedback, our solution keeps growing to meet your specific needs—keeping you ahead of the curve. - Unparalleled Support and Training
More than software providers, we are your technology partners. Our support and training programs are designed to make sure you get the most out of your solution. Whether you need help with a technical issue or just want to learn more about a feature, we’re here to help every step of the way.
With Qualityze Document Management, you can optimize your document processes, reduce costs, and improve overall quality in a cost-effective manner.
Industry Recognitions



Qualityze Delivering Value Across Key Roles
Discover how Qualityze Document Management empowers every key role in managing controlled documents for seamless collaboration and success —from quality managers to regulatory affair manager.
Quality Managers
Quality managers need to ensure that all the controlled documents are in adherence with latest regulatory standards and can be easily accessible for audits/inspections. Qualityze Document Management helps them manage version control, track approvals, and automate reviews while minimizing compliance risks and ensuring audit readiness. Moreover, it creates a culture of quality with consistent documentation practices.
IT Managers
IT managers can rely on Qualityze for secure and centralized storage of the critical documents. The features like role-based access help reduce data silos and maintain document security. The seamless integration with other enterprise systems simplifies document control across platforms, ensuring consistency, security, and better governance.
Regulatory Affairs Managers
Regulatory affairs managers need accurate and up-to-date documents for submissions. Our document management solution makes it easy to keep everything organized and ensures only the most current versions are accessible, complete with clear audit trails for any changes. This helps cut down on errors and delays, making the whole regulatory process more efficient.
Project Managers
Qualityze Document Management gives project managers a central platform to keep track of documents, approvals, and deadlines. It boosts team collaboration by streamlining communication, automating document sharing, and setting reminders for key milestones—making it easier to keep everything on schedule and deliver projects on time.
Legal and Compliance Teams
Legal and compliance teams need quick access to document history, version control, and audit trails. Qualityze makes it simple to stay on top of compliance records, ensuring everything meets regulatory standards and protecting the organization from legal risks. This makes audits and managing disputes a lot less stressful.
HR Managers
HR managers can count on Qualityze Document Management to keep all employee records, training materials, and policy updates securely stored in one place. It makes onboarding and offboarding a breeze, automates policy acknowledgments, and keeps sensitive information protected. Overall, it helps make HR processes smoother and cuts down on tedious admin work
Experience the Qualityze Difference to manage controlled documents with a free demo. Here’s what you’ll get to see in the demo:
We understand that choosing the right document management software is a big decision. That’s why we invite you to experience Qualityze for yourself with a free demo. Here are three compelling reasons to take us up on this offer:
See AI-Powered Document Management in Action
Witness firsthand how our intelligent, AI-driven document management system automates tasks, identifies patterns, and streamlines approval workflows—making your quality management faster and more efficient.
Discover What Your Perfect Document Management System Should Look Like
Experience the Qualityze difference with centralized document storage, seamless collaboration tools, and built-in compliance safeguards. Effortlessly manage version control, track document approvals, and ensure secure access, all in a user-friendly interface tailored to streamline document workflows and support regulatory requirements.
Is It Worth the Hype? Find Out for Yourself!
Don’t just take our word for it. Use the demo to see if Qualityze lives up to its reputation as a leader in the world of Quality. Experience how easy it is to ensure compliance, streamline workflows, and drive continuous improvement.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
- Have better document control
- Maintain security and safety of the documents
- Eliminate errors and have better retention
- Improve decision-making and insigh
Why Document Management Is Important?
Document Management is the foundation for any modern quality management system. It includes the creation, storage, review, and modification of business-critical documents in a systematic and streamlined manner. Such a system enables you to:
How Qualityze transforms your Organization’s Document Management Process into A Winning Process?
Qualityze transforms your organization's document management process into a winning process by offering a robust and reliable software solution. Our cloud-based system eliminates the hassles of traditional document management, including storage limitations. You can easily upgrade your storage as required.
With our best-in-class document management system, you can streamline your entire document lifecycle, from creation to approval, archival to deletion, in a structured manner. Our solution enables you to create a centralized repository for your important documents, ensuring that only authorized individuals can access and assess them. As an administrator, you have control over setting approval dates, publishing dates, deletion schedules, archival periods, and follow-ups well in advance.
By utilizing Qualityze Document Control Management System, your documents are securely stored in a safe vault accessible only to authorized personnel who can modify or delete them. The built-in scheduler simplifies document-related tasks, even when you're not physically present, by sending reminders on specified dates to designate individuals. This ensures timely information delivery to end-users, promoting efficiency and productivity within your organization.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























