Calculate your potential savings with our ROI Calculator
ROI CalculatorCalculate your potential savings with our ROI Calculator
ROI CalculatorStreamline compliance and gain total control over your document lifecycle. Our AI-powered document management software automates workflows, ensures version control, secure access, and provides real-time visibility—so you can focus on quality while staying audit-ready, always.
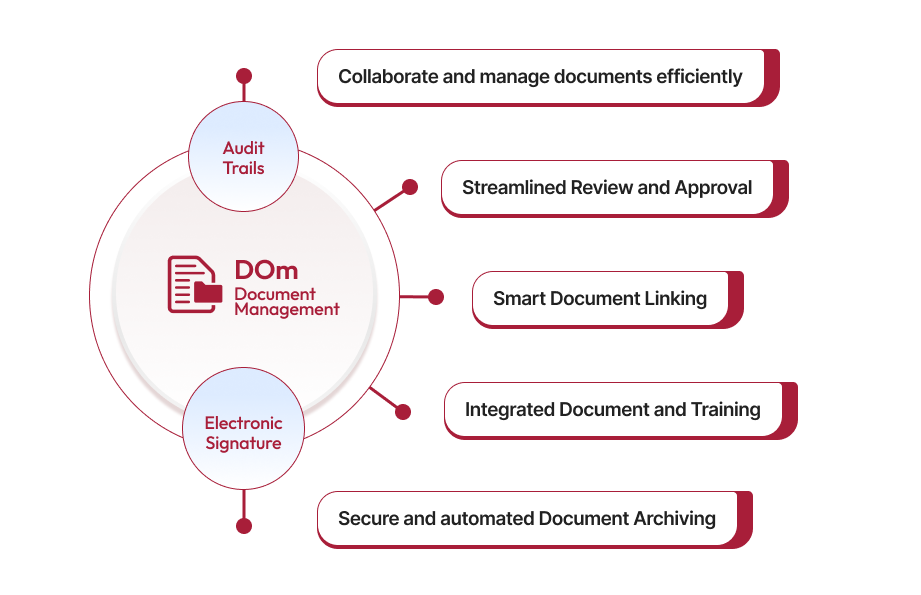
Qualityze Document Management solution simplifies the management of the growing volume of documents by providing a robust framework and convenient sharing and tracking capabilities. With Qualityze, you can streamline your document process and empower your team to achieve document control excellence. When you use Qualityze Document Management solution, you can ensure that your organization stays on top of its documentation needs, reducing the risk of errors and non-compliance.
If you have more questions feel free to reachout to us.
Contact UsDocument Management is the foundation for any modern quality management system. It includes the creation, storage, review, and modification of business-critical documents in a systematic and streamlined manner. Such a system enables you to:
Qualityze transforms your organization's document management process into a winning process by offering a robust and reliable software solution. Our cloud-based system eliminates the hassles of traditional document management, including storage limitations. You can easily upgrade your storage as required.With our best-in-class document management system, you can streamline your entire document lifecycle, from creation to approval, archival to deletion, in a structured manner. Our solution enables you to create a centralized repository for your important documents, ensuring that only authorized individuals can access and assess them. As an administrator, you have control over setting approval dates, publishing dates, deletion schedules, archival periods, and follow-ups well in advance. By utilizing Qualityze Document Control Management System, your documents are securely stored in a safe vault accessible only to authorized personnel who can modify or delete them. The built-in scheduler simplifies document-related tasks, even when you're not physically present, by sending reminders on specified dates to designate individuals. This ensures timely information delivery to end-users, promoting efficiency and productivity within your organization.