Calibration Management Software
Keep Your Equipment Calibrated for Efficient Production Cycles
Achieving consistent quality depends on well-calibrated equipment. Qualityze Calibration Management provides proactive calibration planning, integrated nonconformance management, and real-time insights to ensure your assets are always operating at their best, reducing equipment-related risks and enhancing overall operational efficiency.
Advanced Tolerance Assessment: Reducing Variations, Enhancing Quality
Qualityze Calibration Management offers robust Tolerance Assessment capabilities to identify equipment that falls outside of acceptable limits. Leveraging advanced Repeatability and Reproducibility (R&R) studies, our solution helps you determine whether a Non-Conformance (NC) needs to be initiated. The R&R Study ensures minimal variations in device measurements, maintaining adherence to standard values.

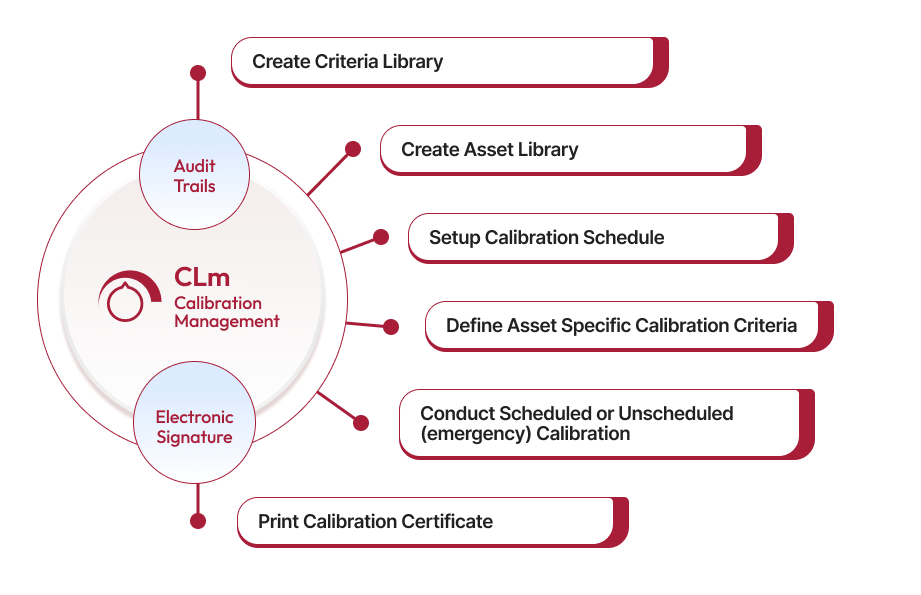
Create Criteria Library for Standardization
The first step in efficient calibration management is to create a comprehensive criteria library. Qualityze Calibration Management allows you to setup 2 types of criteria, variable and attribute criteria for each equipment. This ensures that consistent calibration practices with predefined criteria for measurement, and standard calibration methods are used.

Build an Asset Library for Centralized Management
Managing calibration effectively starts with a complete asset registry. Qualityze allows you to create detailed profiles for each asset, including information such as Asset ID, Type, Location, Last Calibration Date, Maintenance Date and Custodian. Having all asset information centralized helps ensure that calibration activities are scheduled and performed on time and accurately, optimizing equipment availability and reliability.

Setup Calibration Schedules in Advance
To avoid unexpected downtime and ensure that all equipment remains compliant, Qualityze Calibration Management enables you to set up calibration schedules well in advance. These schedules can be configured for different calibration needs and can be assigned to internal teams or external vendors. Advanced scheduling ensures timely calibrations and minimizes disruptions to production.

Define Asset-Specific Calibration Criteria
Qualityze allows you to define asset-specific calibration criteria, including standard measurements and tolerance levels. This step ensures that each asset is calibrated according to its unique requirements, enhancing the precision and accuracy of calibration results. Additionally, you can document R&R (Repeatability & Reproducibility) studies for each asset to ensure consistent performance.

Conduct Scheduled or Unscheduled Calibration
Whether it's a scheduled calibration or an emergency unscheduled calibration, Qualityze supports both. The system generates alerts for upcoming calibrations and also allow you to conduct ad-hoc calibration when unexpected issues arise. Once calibration is complete, actions such as recalibrate, repair, or remove from use can be documented, ensuring all equipment is properly handled and reducing risks associated with inaccurate measurements.

Print Calibration Certificates for Compliance
After a successful calibration, Qualityze allows you to print a calibration certificate that includes details such as the calibration date, due date, and calibration results. This certification process ensures that only properly calibrated equipment is used, meeting regulatory compliance and internal quality standards.

Minimize Risks Through Proactive Calibration Management
Identifying and managing calibration requirements proactively helps minimize equipment-related risks. Our solution enables you to set unique calibration standards for critical assets and manage calibration activities based on asset history and performance. This proactive approach helps in reducing equipment downtime, ensuring reliable performance, and maintaining product quality.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
What Differentiates Us from Others.
We have got you covered for everything you look for in a next generation calibration management solution to be future-ready!
It enables cost-savings through:
- End-to-End Calibration Management
Qualityze helps you manage calibrations at every stage, from initial setup to completion and review. Our system captures all calibration details to ensure accurate and consistent equipment performance. With features like automated calibration schedules, emergency calibration support, and detailed result tracking, we make sure your calibration process is efficient and reliable.
- Configurable to Fit Your Needs
We understand that calibration needs vary across different industries and organizations. Qualityze allows you to tailor calibration workflows, criteria libraries, and asset profiles to meet your specific requirements. Whether you're in pharmaceuticals, aerospace, or general manufacturing, our solution adapts to fit your unique calibration processes.
- Built-In Compliance Tools
Staying compliant with industry regulations can be challenging. Qualityze Calibration Management is designed to support standards such as ISO/IEC and FDA, providing built-in tools to generate calibration certificates, maintain audit trails, and manage electronic signatures. Our compliance features help you meet regulatory requirements seamlessly.
- AI-Powered Insights
Qualityze uses AI to enhance calibration activities. By analyzing historical calibration data, our solution can identify trends, predict potential equipment issues, and provide actionable insights for proactive maintenance. This helps maintain equipment precision, reduce failures, and avoid unnecessary calibration costs.
- Seamless Integration
Qualityze integrates smoothly with existing quality management and enterprise systems, such as ERP and MES systems, to ensure consistent data flow and improve visibility across teams. This seamless integration ensures that calibration processes align well with your overall quality objectives and business systems.
- User-Friendly Interface
Our Calibration Management Solution is designed with a user-friendly interface that simplifies the calibration process. Easy navigation, minimal training requirements, and quick access to critical features help your teams adopt the system effortlessly, reducing downtime and boosting operational efficiency from day one.
- Continuous Improvement Culture
Qualityze supports a culture of continuous improvement by providing insightful analytics and calibration trends. Our system helps teams identify opportunities to enhance equipment reliability, reduce nonconformance, and maintain high standards of quality over time.
- Unparalleled Support and Training
We are committed to your success. Qualityze offers personalized support, comprehensive training, and regular updates to ensure your calibration management system is always performing at its best and meeting your evolving needs.
Industry Recognitions



Qualityze Delivering Value Across Key Roles
Discover how Qualityze Calibration Management helps every key role work better together to maintain equipment accuracy and ensure consistent product quality—from quality assurance managers to calibration technicians.
For Asset Managers
Tracking and managing equipment throughout its lifecycle can be challenging. Qualityze Calibration Management allows Asset Managers to maintain a detailed asset library, including calibration history, status, and location, ensuring that all equipment is properly maintained and operational.
For Calibration Technicians
Calibration Technicians are responsible for performing calibration tasks effectively. Qualityze Calibration Management provides them with clear view of schedules and predefined criteria, making calibration activities easier to manage while minimizing the risk of errors during the calibration process. It also helps them define detailed calibration criteria, including attributes and variables, and conduct Repeatability & Reproducibility (R&R) studies. This ensures that equipment measurements are accurate and meet the required standards.
For Production Managers
Production efficiency is directly affected by equipment reliability. Qualityze Calibration Management helps Production Managers ensure that only well-calibrated equipment is used in production. By minimizing unexpected equipment-related disruptions, it helps keep production schedules on track and maintain product quality.
For Operations Managers
Smooth and efficient operations depend on reliable equipment. Qualityze Calibration Management helps Operations Managers ensure that all equipment is calibrated as scheduled, minimizing unexpected downtime and ensuring efficient, uninterrupted workflows.
For Quality Assurance (QA) Managers
Managing compliance and improving quality can be challenging without an integrated system. Qualityze Calibration Management provides QA Managers with full visibility and control over all calibration activities. They can set up calibration schedules, track nonconformances, and view certificates to ensure regulatory compliance and quality improvement.
For Compliance Officers
Ensuring regulatory compliance requires accurate record-keeping and documentation. Qualityze Calibration Management allows Compliance Officers to track calibration activities, generate calibration certificates, and maintain detailed audit trails, ensuring compliance with standards such as ISO and FDA.
Experience the Qualityze Difference in managing calibration processes with a free demo.
We understand that choosing the right Calibration Management software is a crucial decision for your business. That’s why we encourage you to experience Qualityze in action through a free demo. Here are three compelling reasons to take advantage of this offer:
See AI-Powered Calibration Management in Action
Take a closer look at how our AI-powered Calibration Management simplifies every aspect of the calibration process. From setting up calibration schedules to generating nonconformance records for equipment that is out of tolerance, our intuitive platform helps you manage all calibration activities seamlessly, reducing manual efforts and errors.
Discover What Your Ideal Calibration Management System Should Look Like
Discover how Qualityze offers comprehensive visibility into calibration activities with real-time dashboards, automated notifications, and detailed reports. With our integrated solution, you can ensure that calibrations are conducted efficiently, helping to maintain equipment accuracy, minimize downtime, and ensure compliance with industry standards.
Is It Worth the Hype? Find Out for Yourself!
See for yourself if Qualityze delivers on its promise to transform calibration management. Explore features that make compliance simpler, processes more efficient, and quality control an ongoing success. Experience the capabilities that set Qualityze apart as a leader in the world of quality.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
What is the difference between scheduled and unscheduled (emergency) calibration?
Scheduled calibration involves planned calibration activities that align with a pre-set schedule to ensure equipment accuracy and prevent unexpected issues. Unscheduled (emergency) calibration occurs when there is an urgent need to calibrate equipment, typically due to unexpected deviations or failures. Qualityze's system supports both types of calibration with alerts and predefined criteria for effective management.
How does Qualityze handle equipment that is found to be 'Out of Tolerance'?
When equipment is found to be 'Out of Tolerance' after calibration, Qualityze allows actions such as creating a Nonconformance (NC) report, removing the equipment from use, recalibrating, repairing, or marking it as unrepairable. The system captures details like time taken, cost incurred, asset status, and more, ensuring a comprehensive audit trail.
What information is included in a calibration certificate?
A calibration certificate includes the calibration status, date of calibration performed, and the next due date for calibration. Qualityze ensures these certificates are easily accessible to maintain compliance and verify that only calibrated instruments are used in production.
Can Qualityze Calibration Management integrate with other quality systems?
Yes, Qualityze Calibration Management integrates seamlessly with other quality systems, such as Nonconformance Management. This integration helps streamline calibration management, preventing product recalls and material wastage by ensuring equipment is accurately calibrated before use.
How does Qualityze Calibration Management help in minimizing production downtime?
Qualityze helps minimize production downtime by enabling advanced scheduling of calibration activities, ensuring equipment is calibrated on time to prevent unexpected failures. Its integration capabilities further streamline nonconformance management, reducing potential delays.
What is the role of the criteria library in Qualityze Calibration Management?
The criteria library allows users to define calibration criteria for different equipment attributes and variables. It includes details such as criteria name, measurement values, and tolerance levels, standardizing calibration processes and ensuring equipment meets accuracy requirements.
How can asset-specific calibration criteria be defined using Qualityze?
Qualityze enables users to define asset-specific calibration criteria, including standard measurements and tolerance levels. Users can also document Repeatability & Reproducibility (R&R) studies for each asset, minimizing measurement variations and ensuring consistent quality.
What features does Qualityze Calibration Management offer for equipment record-keeping?
Qualityze provides an asset library for capturing details like Asset ID, type, status, location, and last calibration date. Records are maintained on a centralized platform, simplifying tracking and regulatory audits.
How does Qualityze support compliance with industry standards?
Qualityze Calibration Management is built on a compliance-ready cloud platform, offering features like electronic signatures, audit trails, and validation packs. These tools help organizations meet ISO, FDA, and other regulatory standards by ensuring all calibration activities are well-documented and compliant.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























