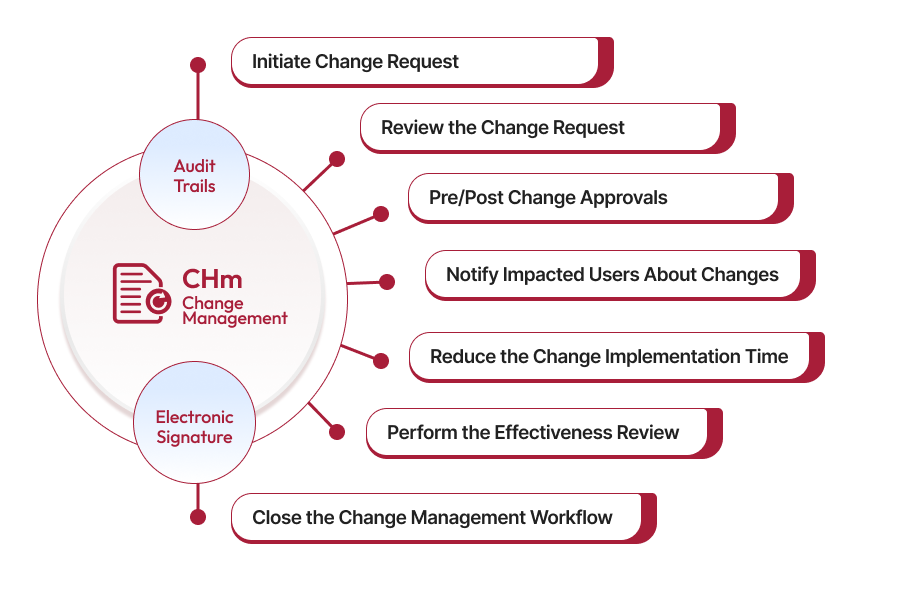
Enterprise Change Management Software
Accelerate Change Processes for Improved Oversight
Handle changes smoothly—from tracking requests to evaluating their impact with Qualityze Change Management. Our intuitive, easy-to-use system helps you reduce risks, stay compliant, and ensure that changes are implemented efficiently, so your organization can keep moving forward without missing a beat.
Intelligent QAI Assistant to Empower Decisions That Drive Change
Seamlessly integrated into the change management software, our decision QAI analyzes trends, anticipates challenges, and offers real-time insights to guide strategic actions. From managing resistance to optimizing workflows, the QAI Assistant empowers leaders with the tools needed to navigate change effortlessly and drive sustainable growth.

Improve Transparency with Change Request Tracking
Easily track and manage change requests from start to finish, making sure everything is clear and everyone is accountable. In today's fast-paced business world, managing changes is very important. Our Change Management system helps you easily track every change request, making the whole process simple and clear. This ensures transparency, accountability, and efficient execution.

Make Informed Decisions with Change Impact Analysis
Perform an impact assessment to understand how changes to systems, processes, and people will impact organization. Conducting change impact assessment provides a complete view of the proposed changes, helping decision-makers see how changes will affect everything. By looking at the effects on systems, processes, and people, you can make smart decisions to reduce problems and get the best results.

Minimize Disruptions with Risk Assessment and Mitigation
Assess the risks that come with changes and create strategies to reduce negative impacts and disruptions. Good change management means finding potential risks, understanding the severity, and making plans to mitigate them. Qualityze helps organizations manage risks ahead of time, so changes happen smoothly without affecting operations too much.

Increase Success with Change Readiness Assessment
Assess your organization’s readiness for change, and identify any gaps that need to be fixed. A change readiness assessment makes sure everyone is prepared for the changes. By identifying potential challenges, gaps and areas that requires additional support, organizations can resolve problems early and make sure the change implementation is successful.

Stay Compliant with Change Approval and Authorization
Ensure that change is reviewed and approved by the right people or groups. With Qualityze’s structured approval process ensures that you only authorize people from required departments, minimizing delays and reduce efforts for every change request.

Drive Continuous Improvement with Performance Monitoring and Measurement
After a change is made, it's important to review its effectiveness. Qualityze helps monitor performance using KPIs and other metrics to see how effective the changes are. This helps with continuous improvement and make your change management processes better over time.

Enhance User Experience with Personalized Dashboards
See all your change-related tasks, reviews, approvals, and actions in real time. Personalized dashboards and reports help you track important metrics related to how well change management is working. Qualityze provides easy-to-use dashboards that fit your needs, making it simple to keep track of activities and progress.

Maximize Efficiency with Seamless Integration
Simplify your workflows and improve efficiency with Qualityze Connect, an integration platform that allows you to seamlessly integrate with your existing systems. Connect your ERP, CRM, EQMS, and Document Management systems with Qualityze Change Management to streamline your change process and ensure compliance.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
What Differentiates Us from Others
We have got you covered for everything you look for in a next generation change management solution to be future-ready!
- End-to-End Change Control
Qualityze offers a well-structured process for managing every aspect of organizational change, from initiation to closure. Through change request tracking, impact analysis, risk assessment, readiness evaluation, approval workflows, and performance monitoring, it ensures that changes are implemented efficiently.
- Configurable to Fit Your Needs
Every business has different needs when managing changes. Qualityze lets you customize workflows, templates, and approval steps to fit your requirements. This flexibility makes sure changes are easy to adopt and fit well into your business.
- Built-In Compliance Tools
Following regulations is very important in change management. Qualityze has built-in tools to make sure every change meets industry standards including FDA. The audit trail and ready-to-go reports, staying compliant is easier.
- AI-Powered Insights Compliance Tools
Qualityze uses AI to do more than just track changes. It finds patterns, spots risks, and gives suggestions to make your change management better. This helps you fix problems before they happen and stay ahead.
- Seamless Integration
Change control works best when all parts of the organization are connected. Qualityze easily connects with your existing systems like ERP and CRM, making sure all information flows smoothly. This helps teams work better together.
- User-Friendly Interface
Managing change can be hard, but your tools shouldn't be. Qualityze has an easy-to-use interface that makes the change process simple. Whether starting or approving a change, your team can finish tasks with little training.
- Continuous Improvement Culture
Good change control is important for continuous improvement. Qualityze helps create a culture where changes are used to help the business grow. By tracking how effective changes are and giving helpful insights, we ensure sustainable success.
- Unparalleled Support and Training
Change control is only as good as the support behind it. Qualityze provides dedicated support, and regular updates to keep your change management system running well. We are committed to your success and always ready to help.
Industry Recognitions



Qualityze Delivers Quality Across Key Roles
Discover how Qualityze Change Management empowers every key role in managing organization wide changes for seamless collaboration and success —from quality managers to regulatory affair manager.
Change Manager
Qualityze makes it easy for Change Managers to handle the change process. It has features like risk management, automated workflows, and real-time alerts. This helps Change Managers keep everything running smoothly, solve problems quickly, and check that changes are made correctly.
Project Manager
Qualityze has an easy-to-use dashboard and tools for Project Managers to work with different teams and track progress. This helps them plan and make changes effectively. Automated alerts and notifications help Project Managers stay on schedule and meet deadlines.
Quality Assurance (QA) Specialist
Qualityze makes reports that are ready for audits and checks all data. This helps QA Specialists make sure changes meet industry standards. By making quality checks simple, Qualityze lets QA Specialists focus on keeping quality high, reducing mistakes, and making sure every change improves products and processes.
IT/Technical Support Team
Qualityze helps the IT and Technical Support Team make technical changes without causing problems for the business. It has strong integration features that help connect different systems and manage changes easily, so everything works well together.
Business Analyst
Qualityze helps Business Analysts understand how changes affect ongoing business processes. With tools for impact analysis, data insights, and reports, analysts make sure changes match business goals and add value. Qualityze AI assistant helps them analyze change trends and make informed decisions to support company growth.
Regulatory Compliance Officer
Qualityze is a validated system with automated workflows, and detailed audit trails to help prepare for audits. Compliance Officers can track each step of the change process to make sure all rules are followed. This helps keep compliance in check and reduces the risk of costly mistakes.
Experience the Qualityze Difference to manage change implementation cycle with a free demo.
We understand that choosing the right change management software is a big decision. That’s why we invite you to experience Qualityze for yourself with a free demo. Here are three compelling reasons to take us up on this offer:
See AI-Powered Change Management in Action
Witness firsthand how our intelligent, AI-driven change management solution automates tasks, identifies patterns, and streamlines processes—making your quality management faster and more efficient.
Discover What Your Perfect Change Management Solution Should Look Like
Explore how Qualityze advanced next generation change control solution provides real-time visibility, intuitive dashboards, and comprehensive reporting tools, giving you complete control over nonconformance events.
Is It Worth the Hype? Find Out for Yourself!
Don’t just take our word for it. Use the demo to see if Qualityze lives up to its reputation as a leader in the world of Quality. Experience how easy it is to ensure compliance, streamline workflows, and drive continuous improvement.
The Start of Something Amazing.
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
What makes Qualityze Change Management Solution different from other change management tools?
Qualityze Change Management Solution is a cloud-based, AI-powered tool that helps companies manage changes better. It has features like the Decision QAI Assistant, pre-defined workflows, risk assessment tools, and easy connections with other systems like ERP, CRM, and EQMS. These features help companies automate changes, make smart decisions using data, and improve communication between teams.
How does the Decision QAI Assistant help in making better change decisions?
The Decision QAI Assistant uses AI to look at quality data, find patterns, and show trends. It helps companies make smart decisions about changes by giving them useful insights into their processes, products, and systems.
What type of changes can be managed using Qualityze Change Management Solution?
Qualityze can help you manage different types of changes, like changes in documents, products, processes, organizational, safety, IT, etc. You can make custom templates for each type of change, including approval steps and workflows, so you can save time and make sure everything is done the same way each time.
How does Qualityze ensure that all changes are effectively tracked and managed?
Qualityze uses a centralized change database to keep track of all change requests, with detailed records to meet regulatory rules. It also gives real-time updates through personalized dashboards, so you can easily see the progress of changes, approvals, and reviews.
How does the solution help assess risks associated with changes?
Qualityze has a Risk Assessment tool that helps look at risks linked to changes. The Risk Priority feature also uses a Risk Assessment Matrix to automatically decide how important each risk is based on how severe it is and how often it might happen.
Can I automate approval processes using Qualityze Change Management Solution?
Yes, Qualityze lets you automate approval workflows for tasks related to changes, like putting changes in place and checking if they work well. This helps make sure that approvals are done quickly and keeps everyone who needs to know informed.
What collaborative features are included in Qualityze Change Management Solution?
The solution includes secure messaging (Chat functionality) for team communication, pre-defined workflows to help manage changes smoothly, and centralized access to change records. This helps your teams to work together to make change implementations successful.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























