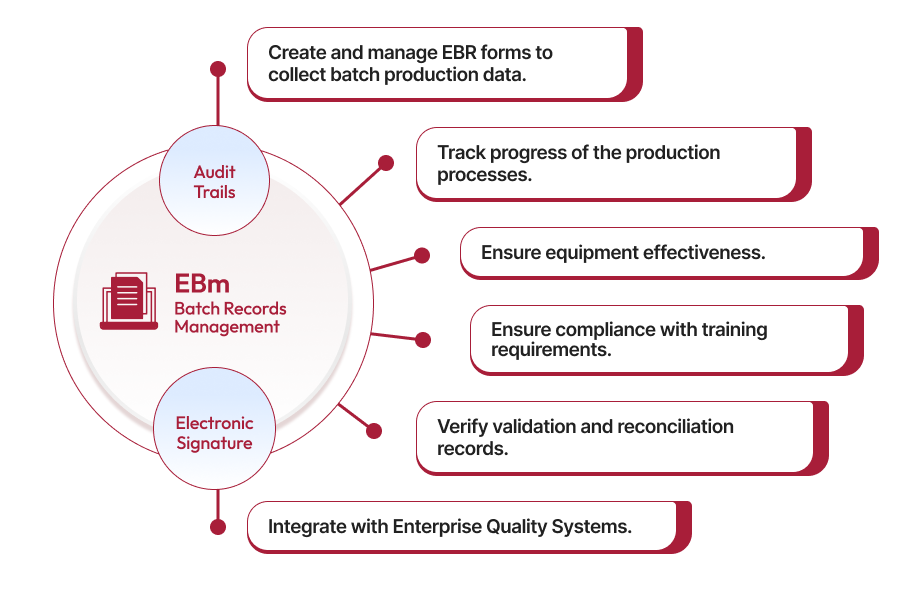
Batch Records Management Software
Achieve Production Excellence with Qualityze Electronic Batch Record Management Solution
Manufacturers worldwide, especially in regulated industries, must ensure products are handled properly throughout production. Qualityze Batch Record Management helps businesses maintain compliance with regulations like CGMP by managing essential production records, such as materials, equipment, and inspections. This cloud-based solution helps manufacturers improve accuracy, detect errors early, and reduce costs. By integrating with existing systems, Qualityze automates data capture, minimizes paper usage, and boosts operational efficiency, enabling companies to scale globally while consistently producing high-quality products.
Enhance Production Efficiency with Real-Time Monitoring and Automation
Qualityze Electronic Batch Record Management Solution enhances production efficiency through real-time monitoring and automation. Track every stage of production—from raw materials to finished goods—to identify bottlenecks and inefficiencies before they cause issues. Automated workflows streamline approvals and data capture, reducing manual effort and minimizing errors. Gain full visibility into operations to optimize resources, maintain quality, and ensure on-time delivery, driving production excellence.

Capture, Manage, and Track All Batch Records in One Place for Better Efficiency
Qualityze makes it easy to collect and manage all the important information about batch records, like production data, equipment used, operator details, material lists, key dates, and analysis. All of this is stored in one secure centralized platform. This makes it simple to find what you need, reduces paperwork, makes work more efficient, and ensures no important details are missed.

Monitor Production Processes to Prevent Quality Problems
Keeping an eye on production is key to making good-quality products. With Qualityze, you can track all the important production details to make sure they meet quality standards. This helps find and fix issues before they become bigger problems, keeping your product safe and following the rules. You can track problems like equipment failures and other production issues to solve them quickly.

Track Inventory and Equipment Status for Better Production
Qualityze helps you see everything about inventory and equipment status, so you can manage raw materials, check equipment, and schedule maintenance. This includes tracking material amounts, expiration dates, and equipment maintenance schedules. By keeping everything organized, you can make production more efficient, reduce downtime, and avoid quality issues.

Improve Teamwork for Faster Problem Solving
Good teamwork is important for smooth production. With Qualityze, everyone can access the same batch records, which helps different departments work together to solve problems faster. The platform makes it easy for production, quality, and procurement teams to communicate, making sure everything runs smoothly. Qualityze also gives different people the right level of access, so they can see what they need to do their jobs.

Adhere to Compliance with Automated Workflows
Qualityze helps you follow important rules by automating things like production approvals, electronic signatures, and audit trails. This helps you stay compliant with regulatory standards like FDA, CGMP, GAMP5, and ISO standards. Automated reminders for approvals and audits make it easier to meet deadlines and avoid penalties.

Easily Manage Batch Records for Compliance and Audits
Managing batch records can be complicated, but Qualityze makes it simple. With ready-made templates and a centralized storage system, all batch records are kept secure, easy to access, and ready for audits. The built-in audit trail keeps everything transparent, making it easier to stay compliant throughout the product's lifecycle. Qualityze also lets you configure forms to fit your business needs, making it flexible.

Integrate with Other Quality Systems for Better Quality Management
Qualityze works well with other quality systems like CAPA, Training Management, Inspection Management, and Nonconformance Management. This creates a closed-loop system that helps track and improve quality processes, making batch records more accurate, reducing risks, and supporting continuous quality improvement. It also integrates with systems like ERP and MES to make production more visible and efficient.

Keep Batch Record Data Safe
Security is very important when handling sensitive production data. Qualityze uses advanced data encryption, role-based access, and audit trails to make sure all batch record information is kept secure and only accessed by authorized people. This cloud-based system helps companies meet compliance requirements while keeping their data safe. The centralized storage makes it easy to find records, especially during audits.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
We have everything you need for a next-generation Batch Records Management solution to be future-ready!
Key benefits:
- End-to-End Calibration Management
Qualityze helps you manage calibrations at every stage, from initial setup to completion and review. Our system captures all calibration details to ensure accurate and consistent equipment performance. With features like automated calibration schedules, emergency calibration support, and detailed result tracking, we make sure your calibration process is efficient and reliable.
- End-to-End Batch Record Management
Qualityze enables you to manage the entire lifecycle of batch records—from creating custom EBR forms to tracking production progress and maintaining compliance. Our solution ensures that every detail of your production process is accurately documented, reducing errors and ensuring efficient batch management.
- Configurable to Fit Your Needs
Qualityze Batch Record Management is fully configurable to meet your unique business requirements. Tailor fields, templates, and workflows to match your production processes and industry standards. Qualityze adapts to your needs, making batch management smooth and effective.
- Built-In Compliance Tools
Stay ahead of evolving regulations with Qualityze built-in compliance tools. Our solution helps you automatically track the latest regulatory requirements, such as FDA and GMP, and adjust your batch record processes accordingly. This proactive approach ensures ongoing compliance while minimizing manual effort.
- Built-In Risk Quality Tools
Qualityze includes advanced quality tools to ensure product consistency and minimize risks. With built-in validation checks, data integrity measures, and reconciliation processes, potential production issues can be identified, addressed, and resolved before they escalate, supporting a culture of quality, safety, and operational excellence.
- Seamless Integration
Qualityze integrates seamlessly with existing enterprise systems, such as ERP, CAPA, and Document Management systems. This ensures all batch management processes are connected, helping you manage production quality efficiently without disrupting operations.
- User-Friendly Interface
Qualityze Batch Record Management features an intuitive, user-friendly interface that requires minimal training. The cloud-based solution allows you to manage batch records anytime, anywhere, while advanced search capabilities help you access crucial production data quickly.
- Continuous Improvement Culture
Qualityze helps you create a closed-loop system to ensure continuous quality improvement. Our solution empowers your team to monitor, evaluate, and enhance batch record processes consistently, promoting long-term excellence and resilience in managing production quality.
- Unparalleled Support and Training
Our team provides exceptional support with personalized training sessions, customer assistance, and regular software updates to keep your batch record management efficient. Qualityze empowers your organization to continuously improve while staying compliant with evolving regulatory standards.
With Qualityze EBR Management Software, you can optimize your batch record management processes, achieve higher quality standards, and realize substantial cost savings in your operations.
Industry Recognitions



EBR management benefits to various operational functions
Discover how Qualityze Electronic Batch Record Management Software helps different roles streamline production, ensure compliance, and enhance efficiency—from Production Managers to Quality Control Analysts.
For Production Managers
Production Managers make sure the whole production process goes smoothly. Qualityze EBR Management helps them see the progress of batches in real time, track production, find problems, and fix them quickly. With features like tracking equipment and inventory, Production Managers can make sure resources are ready, avoid delays, and keep things efficient.
For Quality Assurance (QA) Managers
QA Managers make sure products meet quality standards and follow rules. Qualityze EBR Management has automated workflows, electronic signatures, and centralized data storage to make reviewing and approving batch records easier. With detailed audit trails, QA Managers can check records, reduce mistakes, and keep product quality high.
For Regulatory Compliance Specialists
Regulatory Compliance Specialists make sure all production records meet regulatory standards. Qualityze EBR Management helps them by automating compliance tasks, capturing production data accurately, and keeping audit-ready records. This reduces manual work, makes sure the rules are followed, and makes audits easier.
For IT and System Administrators
IT and System Administrators set up and secure the EBR system. Qualityze EBR Management works well with other enterprise systems like ERP and CRM, creating a smooth workflow. Administrators can control data access and security, keeping sensitive information safe. This integration helps manage data better and reduces security risks.
For Production Operators
Production Operators record batch data during production. Qualityze EBR Management makes data entry easy with customizable forms and simple user interfaces. Operators can use pre-defined templates to record process details, equipment use, and material amounts accurately, reducing errors and making sure the right information is recorded.
For Quality Control (QC) Analysts
QC Analysts test and analyze product batches to make sure they meet quality standards. Qualityze EBR Management gives QC Analysts easy access to batch records and test data, helping them track results and spot quality issues. This helps them respond quickly, coordinate corrective actions, and make sure only good-quality products move forward in production.
Experience the Qualityze Difference in Batch Records Management with a Free Demo.
We understand that choosing the right Batch Record Management system is a crucial decision for your business. That's why we invite you to see Qualityze in action through a free demo. Here are three compelling reasons to take advantage of this offer:
See Next Generation Batch Records Management in Action
Discover how our advanced solution makes managing Electronic Batch Records (EBR) simple and efficient. From creating custom batch record forms to tracking production progress, Qualityze offers a powerful, centralized platform that reduces manual work, improves data accuracy, and ensures real-time visibility across your entire production process.
Discover What Your Ideal Batch Records Management System Should Look Like
Qualityze seamlessly integrates batch record management with other quality processes like CAPA, Document Management, and Supplier Quality Management. Our advanced integration capabilities give you a unified approach to maintaining compliance, managing risks, and streamlining production workflows. Track production details, manage approvals, and maintain accurate records—all from one secure cloud-based platform.
Is It Worth the Hype? Find Out for Yourself!
See how Qualityze helps you ensure compliance, improve production efficiency, and maintain the highest standards of product quality. Our platform is designed to simplify batch management, enhance data security, and provide a strong return on your investment. Experience the capabilities that set Qualityze apart as a leader in the world of quality.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
What are the key features of the Qualityze Batch Record Management System, and how do they help production management?
Qualityze Batch Record Management System has features like configurable EBR forms, a centralized batch record database, and inventory management tools. With configurable forms, you can create custom electronic batch records, like Blend Production Records and Process Checklists, to collect consistent data during production. The centralized batch record database keeps all records in one place, making it easy to track and meet regulatory standards.
How does the Decision QAI Assistant help improve quality during batch management?
The Decision QAI Assistant analyzes production batch records to identify patterns, trends, and insights. This helps you find ways to improve, prevent recurring issues, and make better decisions.
How does Qualityze make sure Electronic Batch Records (EBRs) meet industry standards and are ready for audits?
Qualityze Batch Record Management System makes sure records follow industry standards by keeping a detailed audit trail for each record. It logs every activity, such as form names, bulk orders, and inspections, with timestamps and user details. This makes sure that records are traceable and easy to access during audits. The system also uses electronic signature validation to keep records safe and authentic, meeting standards like FDA 21 CFR Part 11 and GMP.
How does Qualityze track equipment and inventory to avoid downtime and make production more efficient?
Qualityze Batch Record Management System has tools for tracking equipment and inventory. It monitors equipment health, such as calibrations and maintenance schedules, to make sure they are ready when needed. It also keeps track of materials used in production, including expiration dates and quality, to prevent problems. By managing line/room clearance activities, it also helps reduce the risk of contamination. These features work together to reduce downtime and keep production running smoothly.
What collaboration and workflow tools are available in Qualityze Batch Record Management System, and how do they help teams work better?
Qualityze has several tools to help teams work better. Pre-defined EBR form templates make sure that data is collected consistently. The task scheduler helps users assign and keep track of tasks in advance, reducing mistakes. The Chatter feature allows real-time messaging, so team members can solve issues quickly. Automated approval workflows keep everyone informed about pending tasks, and electronic signatures help secure the approval process.
How does Qualityze keep electronic batch records secure and prevent unauthorized access?
Qualityze uses multiple security features to protect electronic batch records. Access controls make sure that only authorized people can access the records, based on their roles. Data is encrypted both when stored and during transmission, keeping it safe from unauthorized access. Qualityze also has integrated reporting to track changes, making sure data stays secure and intact.
What are the different types of reports one can generate with Qualityze Batch Record Management System, and how do they help monitor production and meet compliance?
Qualityze allows users to create different reports, providing insights into production trends, equipment health, inventory, and more. These reports help you identify problems, improve quality control, and meet compliance requirements. For example, reports can show where equipment needs maintenance or where there are inventory discrepancies. These insights make it easier to pass audits and fix issues quickly.
Can Qualityze Batch Record Management System work with other systems we already use, and how does that help?
Yes, Qualityze Batch Record Management System can connect with other systems like ERP (Enterprise Resource Planning), CRM (Customer Relationship Management), and EQMS (Enterprise Quality Management System). This integration makes it easier to manage production, quality, and compliance by having everything in one workflow. It reduces manual data entry, prevents errors, and makes sure all data is up to date, leading to better decision-making and efficiency.
How does Qualityze improve the user experience and provide real-time visibility for batch record tasks?
Qualityze gives each user personalized dashboards, showing real-time updates on tasks, reviews, and approvals. This helps users focus on critical tasks and meet production goals on time. Features like electronic signature validation and alerts make sure everyone stays informed and engaged in the process, keeps everything compliant with less effort.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























