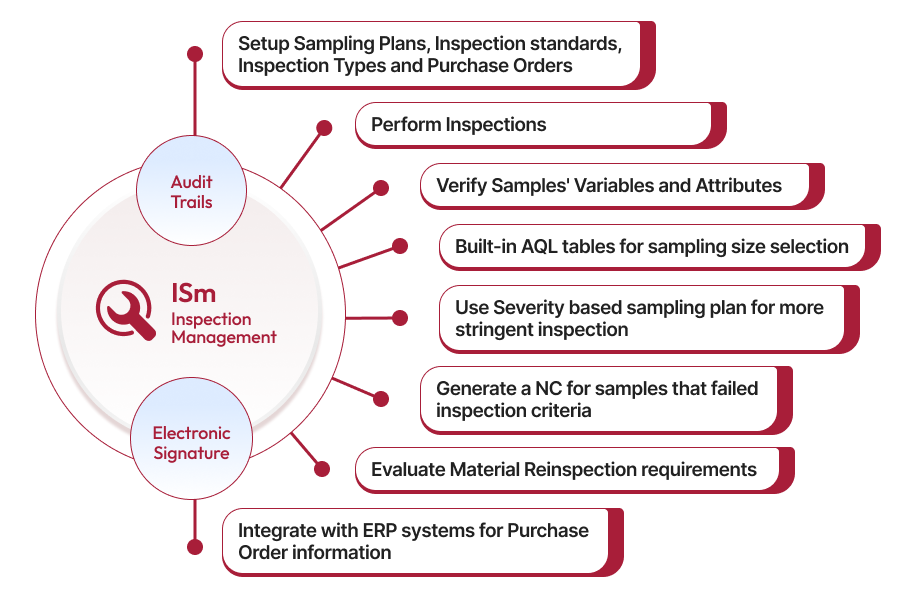
Inspection Management Software
Accelerate Inspection Cycles for Greater Operational Efficiency
Speed up your inspection processes while gaining complete visibility into each stages of the inspection process. Our AI-powered inspection management solution eliminates bottlenecks by automating routine tasks, ensuring compliance with regulations, and offering real-time data that helps your team conduct inspections more effectively allowing for a quicker decision.
Intelligent QAI Assistant to Ensure Timely Inspections
Our QAI assistant delivers real-time insights, automates routine inspection tasks, and accelerates inspection processes by prioritizing inspections. This ensures more efficient inspections, reduces delays, and helps maintain high-quality standards across all operations.

Simplify Material Inspections with Ready-to-Use Templates
Efficient inspection management begins with standardized processes. Qualityze Inspection Management System offers ready-to-use templates that streamline material inspection from initiation to completion. These templates ensure that all inspections follow a consistent format and process, saving time and reducing errors throughout the inspection lifecycle.

Quick and Easy Inspection Documentation
Recording inspection details accurately is key to effective inspection management. Qualityze makes it easy to capture crucial information like product details, inspection type, sample size, on time/late delivery, shortage/overage of materials, supplier data, and lot numbers. With pre-configured fields and customization options, Qualityze ensures all necessary data is recorded efficiently, maintaining compliance and accuracy.

Classify and Prioritize Inspections for Better Quality Control
Once an inspection is initiated, it's essential to classify and prioritize it based on risk and quality requirements. Qualityze provides tools to categorize inspections by product type, severity, criticality and supplier history. This helps quality teams focus on the most critical inspections, ensuring that higher-risk materials are managed effectively, promoting better quality outcomes.

Take Action with Severity-Based Sampling Plans
Ensuring thorough inspection is key to preventing quality issues. Qualityze enables you to create severity-based sampling plans to handle materials that require more rigorous inspection. Using Acceptable Quality Level (AQL) or Lot Tolerance Percent Defective (LTPD) standards, Qualityze helps your team carry out stringent inspections efficiently, ensuring compliance and reducing risks.

Automate Nonconformance Records for Seamless Transition
If an inspection reveals defects, creating a nonconformance (NC) record is easy with Qualityze. With a single click, you can initiate NC records for samples that fail to meet quality criteria, ensuring seamless transitions between inspection and nonconformance management processes. This automation reduces manual tasks, keeps productivity high, and maintains traceability throughout the quality control process.

Evaluate Material Reinspection Requirements
Qualityze Inspection Management also helps you determine if reinspection is needed for materials that did not meet quality standards initially. Whether due to minor issues or uncalibrated measurement devices, reinspection can be scheduled to verify quality before materials enter production. This feature helps reduce rework and waste, ensuring only high-quality materials are accepted.

Real-Time Visibility and Control with Dashboards
Qualityze dashboards provide real-time updates on inspection progress, allowing stakeholders to track key metrics such as inspection completion rates, nonconformance trends, and quality performance. These dashboards help decision-makers spot potential delays, prioritize inspections, and make data-driven decisions to support continuous quality improvement.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
What Differentiates Us from Others We have got you covered for everything you look for in a next generation inspection management solution to be future-ready!
Qualityze enables you to:
- End-to-End CAPA Management
Our solution offers an end-to-end CAPA management framework, guiding you from problem identification, investigation, verification, and resolution of quality issues. Every aspect—from root cause analysis to preventive measures—is tracked, managed, and verified within one system. - End-to-End Inspection Management
Qualityze helps you manage inspections at every step, from incoming materials to final production checks. Our system keeps track of all the details to ensure inspections are thorough and efficient. With features like real-time tracking, customizable sampling plans, and automated workflows, we make sure your inspection process runs smoothly. - Configurable to Fit Your Needs
We know that every company is different. Qualityze lets you customize inspection workflows, sampling templates, and inspection standards to fit your needs. Whether you're in healthcare, manufacturing, or any other industry, our system adjusts to work for you. - Built-In Compliance Tools
Some industries have strict rules, and staying compliant can be tough. Qualityze Inspection Management is built to meet important standards like ISO 9001, FDA, and EU Annex 11. Our built-in compliance tools make it easy to access reports, track workflows, and create audit trails so you can stay compliant. - AI-Powered Insights
Qualityze uses AI to do more than just basic inspections. Our system looks at past inspection data to find patterns, analyze supplier performance, loosen or tighten the inspection scope, and suggest prioritization. This helps you keep quality high and avoid costly mistakes. - Seamless Integration
Qualityze works well with your existing systems, like ERP and supply chain tools, so sharing data is easy and teams have better visibility. This integration helps make sure your inspection processes fit smoothly with your other quality goals. - User-Friendly Interface
We designed Qualityze to be easy to use. Unlike other complex systems, our simple interface means your quality teams can start using it quickly without much training. This helps reduce downtime and boosts efficiency right away. - Continuous Improvement Culture
Qualityze doesn't just help with inspections—it helps you learn from them. By tracking trends and giving useful analytics, our system helps your team keep making the product better, reduce defects, and keep quality standards high. - Unparalleled Support and Training
We want you to succeed. Qualityze offers personalized support, ongoing training, and regular updates to make sure your inspection management system stays up to date and works its best. - Global Regulatory Reporting
Staying compliant with global rules can be hard. Qualityze makes it simple with ready-made forms and automated workflows for regulatory bodies like FDA, ISO, and other standards. This way, you get accurate reports on time and stay compliant easily.
Industry Recognitions



Qualityze Delivering Value Across Key Roles
Discover how Qualityze Inspection Management helps every key role work better together to manage inspections and get great results—from quality leaders to regulatory specialists.
For Quality Control Inspectors
We know that manual inspections can be tough and lead to mistakes. Qualityze makes inspection tasks easier by giving you simple steps to check incoming materials, production stages, and pre-shipment inspections. You can easily capture and document all the inspection details to make sure only good quality materials move forward, reducing quality issues.
For Quality Assurance Managers
Staying compliant and improving quality can be hard when systems don’t connect well. Qualityze helps by giving you full visibility and control over all inspections. From managing sampling plans to looking at real-time data, our system makes sure you follow all the rules and keep improving quality.
For Procurement Officers
Unreliable suppliers can cause delays and increase costs. Qualityze helps you make better purchasing decisions by giving you information on supplier performance based on inspection results. This way, you can keep only the best suppliers, reduce risks, and maintain high quality.
For Production Managers
Production delays and costly rework often happen because of poor-quality materials. Qualityze Inspection Management gives you real-time insights to make sure only defect-free materials reach the production line. This helps keep production on schedule, reduce delays, and improve product quality.
For Regulatory Compliance Officers
Following industry regulations means keeping careful records. Qualityze helps you track all inspection activities with features like audit trails, electronic signatures, and detailed reports. This way, you can meet all regulatory requirements, including ISO 9001, FDA, and EU Annex 11, with ease.
For Warehouse Managers
Handling incoming materials correctly is key to good product quality. Qualityze Inspection Management helps you manage inspections smoothly so that only approved materials go into inventory. This reduces the risk of poor-quality materials entering production and helps you align with quality teams.
For Executive Management (C-Level)
Your goal is to make strategic decisions that improve operations. Qualityze gives you full visibility into inspection processes with real-time dashboards and reports. Track important quality metrics, find areas to improve, and make decisions that reduce risks and boost efficiency.
For Supplier Quality Managers
Supplier performance affects product quality. Qualityze lets you set inspection standards for suppliers, automate sampling plans, and assess supplier performance using past data. This helps ensure only high-quality materials are sourced, reducing risks and costs from poor quality.
Experience the Qualityze Difference to manage quality inspections with a free demo. Here’s what you’ll get to see in the demo:
We understand that choosing the right Inspection Management software is a crucial decision for your business. That’s why we encourage you to experience Qualityze in action through a free demo. Here are three compelling reasons to take advantage of this offer:
See AI-Powered Complaints Management in Action
Take a closer look at how our AI-powered Inspection Management simplifies every aspect of the inspection process. From setting up sampling plans to generating nonconformance records for failed inspections, our intuitive platform helps you manage all inspection activities seamlessly, reducing manual efforts and errors.
Discover What Your Perfect Inspection Management System Should Look Like
Discover how Qualityze offers comprehensive visibility into inspection activities with real-time dashboards, automated notifications, and detailed reports. With our integrated solution, you can ensure that inspections are conducted efficiently, helping to maintain product quality and compliance while mitigating supplier risks.
Is It Worth the Hype? Find Out for Yourself!
See for yourself if Qualityze delivers on its promise to transform inspection management. Explore features that make compliance simpler, processes more efficient, and quality control an ongoing success. Experience the capabilities that set Qualityze apart as a leader in the world of quality.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
What is Qualityze Inspection Management, and how can it help my business?
Qualityze Inspection Management is a cloud-based software solution that helps streamline, standardize, and manage inspection processes throughout different manufacturing stages. Whether conducting incoming, in-process, or pre-shipment inspections, Qualityze simplifies the process, making it easier to verify product quality before releasing it to consumers. It comes pre-loaded with internationally recognized standards such as ANSI/ASQC Z1.4 (ISO 2859-1), allowing you to ensure compliance while reducing the risks and costs associated with poor quality.
How does Qualityze Inspection Management help ensure product quality and regulatory compliance?
Qualityze Inspection Management helps establish robust inspection processes, ensuring product quality by providing tools to define sampling plans, inspection standards, and acceptance thresholds. It enables inspections across incoming materials, production stages, and pre-shipment checks. The system is also compliant with regulatory standards such as ISO 9001, FDA 21 CFR Part 11, and EU Annex 11, offering features like electronic records, e-signatures, and audit trails to maintain transparency and compliance.
Explain the different types of inspections that can be performed using Qualityze Inspection Management.
With Qualityze Inspection Management, you can perform multiple types of inspections, including incoming inspections (materials arriving at the facility), production inspections (during the manufacturing process), and pre-shipment inspections (before final delivery to the customer). This holistic approach ensures proper quality management at all stages, reducing the likelihood of defects and minimizing the risk of non-conformance.
What are the key features of Qualityze Inspection Management?
Key features of Qualityze Inspection Management include: - Sampling Plans: Supports ANSI/ASQC Z1.4 and custom sampling plans to identify sample size and acceptance thresholds. - Inspection Standards: Define inspection standards for different types of inspections like parts, suppliers, or products. - Switching Rules: Automatically switch between different inspection levels (normal, tightened, reduced) based on historical data. - Nonconformance Management Integration: Allows you to create Nonconformance (NC) records to manage quality issues identified during inspections. - ERP Integration: Seamlessly integrates with existing ERP systems for better tracking of purchase orders and material inspection.
How does Qualityze Inspection Management integrate with existing IT systems?
Qualityze Inspection Management integrates seamlessly with your existing IT ecosystem, such as ERP systems, to complement your supply chain processes. Integration enables the use of purchase orders, work orders, and lot information directly during inspections, and the inspection data can be sent back to the ERP system for further actions, such as stock realization after successful inspections.
How can Qualityze Inspection Management help mitigate supplier risk?
Qualityze Inspection Management provides advanced tools to help identify critical suppliers and mitigate risks. You can define different inspection standards, sampling plans, and severity-based AQL (Acceptable Quality Level) for each supplier based on their performance history. The automated switching rule adjusts inspection levels based on the supplier's performance, helping reduce risks and ensuring that only high-quality materials are used in production.
What are severity-based sampling plans, and how do they work in Qualityze Inspection Management?
Severity-based sampling plans in Qualityze Inspection Management are used to enforce more stringent inspections of critical criteria of materials. With configurable workflows and forms, users can define AQL (Acceptable Quality Level) levels for each criterion based on its criticality, ensuring that only high-quality materials are allowed into production. This approach helps reduce product failures and guarantees compliance with industry standards.
How does Qualityze Inspection Management support different inspection standards and attributes?
Qualityze Inspection Management allows you to create multiple inspection standards that fit your organization’s needs. It categorizes inspection characteristics into attributes (such as pass/fail) and variables (nominal values and tolerances). With the in-built integration of Qualityze Document Management, you can easily document and reference drawings, SOPs, or any other related documents for seamless inspections.
What industries can benefit from Qualityze Inspection Management?
Qualityze Inspection Management is designed to cater to various industries, including Hospitality, Manufacturing, Life Sciences, Pharmaceuticals, Medical Devices, Cannabis, Nutraceuticals, Biologics, Biotech, Food & Beverages, Automotive, Aerospace, Defense, and Logistics. It provides industry-specific inspection capabilities, ensuring compliance with applicable regulatory standards, and helps organizations manage quality inspections efficiently, regardless of the industry.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























