


Calculate your potential savings with our ROI Calculator
ROI Calculator
The life sciences industry is a sector where winning equates to being exact, safe, and following the rules. It is an are...

In life sciences and manufacturing, the integrity of your final product is only as strong as your most vulnerable extern...
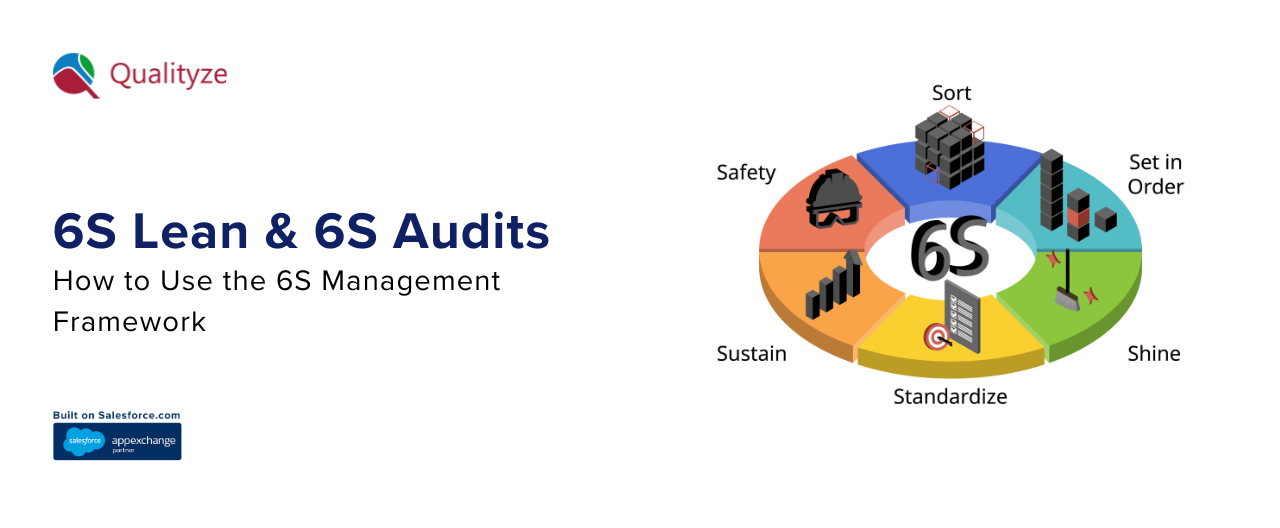
The 6S framework basically consists of six simple yet effective ideas: Sort (Seiri), Set in Order (Seiton), Shine (Seiso...

In modern quality management, the integrity and security of documentation are paramount, especially for regulated indust...

In today’s hyper-connected global marketplace, the effective management of vendor relationships—known as Supplier Perfor...

Digital transformation has hit the factory floor, yet many quality systems lag behind. Learn how expert QMS Workflow Des...

The pharmaceutical supply chain is a web of risk; only robust pharma recalls track and trace solutions can offer the nec...

Every organization striving for operational excellence eventually faces one central truth — a Quality Management System ...

In regulated sectors, a quality management system is the pillar of patient safety as well as business sustainability. Gr...

The promise of a modern eQMS is efficiency, speed, and bulletproof compliance. The reality? Many organizations crash int...

In the life sciences, medical devices, and manufacturing sectors, the effectiveness of your Quality Management System is...

Every Quality Assurance professional knows that effective GMP labeling is a primary defense against product recalls. The...